In organic chemistry, inter- and intramolecular coupling reactions of aromatic substrates represents a contemporary hot topic. In our research group we are focusing on the application of molybdenum(V) reagents in oxidative coupling reactions of electron-rich aryl moieties.
MoCl5 - a Versatile Reagent
We found that molybdenum pentachloride, a one-electron oxidant (if no additional lewis acid is present),[1] proves to be an inexpensive and powerful alternative to frequently used transition metal salts. This reagent was successfully applied in various oxidative coupling reactions. The intramolecular approach revealed the formation of a library of cyclic architectures like five-,[2] six-,[3] seven-,[4] and eight-membered[5] ring systems. In addition, also spiro-cyclic derivatives[3d, 6] and heteronuclear structures[7] could be accomplished. The intermolecular access towards triphenylene ketales ended up in a novel C3-symmetrical receptor for supramolecular recognition.[8] MoCl5 can also be used as a clorination reagent of phenoxyacetate derivatives.[9] In contrast to transition metal catalyzed cross-coupling reactions, strong electrophiles such as iodo substituents are tolerated in this type of transformation. Even an all-ortho-tetraiodo biphenol could be directly accessed.[10] For para-substituted arenes, the steric bulk of alkyl groups and the activating effect of methoxy functional groups provides a distinct ortho-selectivity.[8e]
Mechanistic considerations
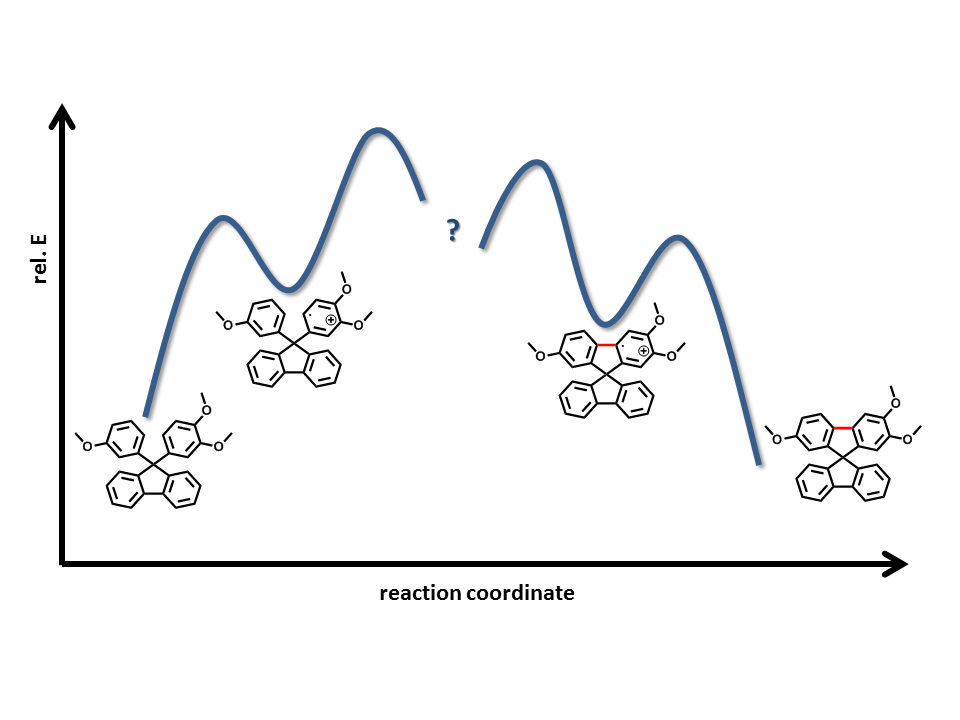
To understand the reactivity and selectivity of molybdenum(V) reagents, we started the investigation of reaction intermediates. By the application of different techniques (EPR, DFT) we found that the initial reaction step is a single electron transfer (SET) from the activated arene to the Mo(V) center atom which takes place by an inner sphere mechanism.[11] Together with collaboration partners, we found, by ESI-MS and DFT calculation, that the pre-last step is an over-oxidized species, which will be reduced in a subsequent aqueous work-up.[1]
 Novel Mo(V) Reagents
Novel Mo(V) Reagents
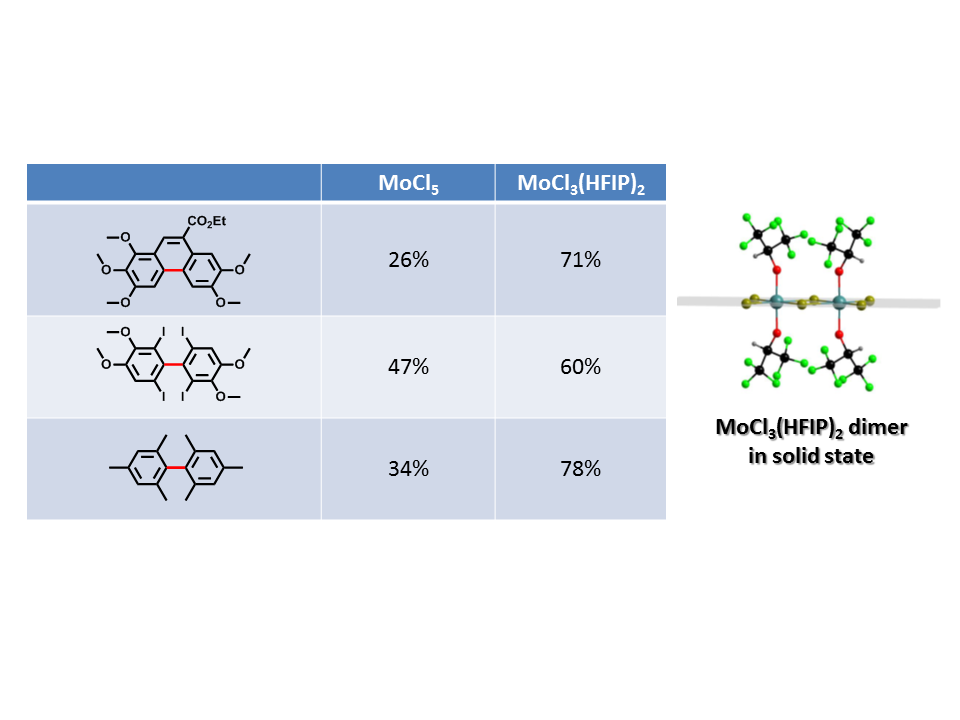
Even though molybdenum pentachloride is already an outstandingly powerful reagent, the avoidance of by-products deserves further investigations towards novel reagents. The successful substitution by 1,1,1,3,3,3-hexafluoroisopropanol resulted in a more reactive and selective reagent respectively.[12]
 SeO2 – an Old Reagent in a New Fashion
SeO2 – an Old Reagent in a New Fashion
Selenium dioxide (SeO2) is known over a long time in organic chemistry for oxidative transformations. We recently targeted the formation of either biphenols or diaryl selenides by a solvent depended approach.[13] The choice of either acetic acid or pyridine yielded in the respective phenolic derivatives. Additionally, the use of 1,1,1,3,3,3-hexafluoroisopropanol provided biphenols in a cross-coupling fashion.[14]
Lit.:
[1] M. Schubert, P. Franzmann, A. Wünsche von Leupoldt, K. Koszinowski, K. Heinze, S. R. Waldvogel, Angew. Chem. Int. Ed. 2016, 55, 1156-1159. [2] P. Franzmann, S. Trosien, M. Schubert, S. R. Waldvogel, Org. Lett. 2016, 18, 1182-1185. [3] (a) S. Trosien, S. R. Waldvogel, Org. Lett. 2012, 14, 2976-2979; (b) M. Schubert, S. Trosien, L. Schulz, C. Brandscheid, D. Schollmeyer, S. R. Waldvogel, Eur. J. Org. Chem. 2014, 2014, 7091-7094; (c) K. Wehming, M. Schubert, G. Schnakenburg, S. R. Waldvogel, Chem. Eur. J. 2014, 20, 12463-12469; (d) M. Schubert, K. Wehming, A. Kehl, M. Nieger, G. Schnakenburg, R. Fröhlich, D. Schollmeyer, S. R. Waldvogel, Eur. J. Org. Chem. 2016, 2016, 60-63. [4] (a) B. Kramer, S. R. Waldvogel, Angew. Chem. Int. Ed. 2004, 43, 2446-2449; (b) K. Hackeloer, G. Schnakenburg, S. R. Waldvogel, Org. Lett. 2011, 13, 916-919; (c) K. Hackelöer, S. R. Waldvogel, Tetrahedron Lett. 2012, 53, 1579-1581. [5] (a) B. Kramer, A. Averhoff, S. R. Waldvogel, Angew. Chem. Int. Ed. 2002, 41, 2981-2982; (b) B. Kramer, R. Fröhlich, Siegfried R. Waldvogel, Eur. J. Org. Chem. 2003, 2003, 3549-3554. [6] S. Trosien, D. Schollmeyer, S. R. Waldvogel, Synthesis 2013, 45, 1160-1164. [7] S. Trosien, P. Böttger, S. R. Waldvogel, Org. Lett. 2014, 16, 402-405. [8] (a) S. R. Waldvogel, R. Fröhlich, C. A. Schalley, Angew. Chem. Int. Ed. 2000, 39, 2472-2475; (b) S. R. Waldvogel, Synlett 2002, 2002, 0622-0624; (c) N. M. Boshta, M. Bomkamp, G. Schnakenburg, S. R. Waldvogel, Chem. Eur. J. 2010, 16, 3459-3466; (d) N. M. Boshta, M. Bomkamp, G. Schnakenburg, S. R. Waldvogel, Eur. J. Org. Chem. 2011, 2011, 1985-1992; (e) D. Mirk, B. Wibbeling, R. Fröhlich, S. R. Waldvogel, Synlett 2004, 2004, 1970-1974. [9] D. Mirk, O. Kataeva, R. Fröhlich, S. R. Waldvogel, Synthesis 2003, 2003, 2410-2414. [10] S. R. Waldvogel, E. Aits, C. Holst, R. Frohlich, Chem. Commun. 2002, 1278-1279. [11] J. Leppin, M. Schubert, S. R. Waldvogel, K. Heinze, Chem. Eur. J. 2015, 21, 4229-4232. [12] M. Schubert, J. Leppin, K. Wehming, D. Schollmeyer, K. Heinze, S. R. Waldvogel, Angew. Chem. Int. Ed. 2014, 53, 2494-2497. [13] T. Quell, M. Mirion, D. Schollmeyer, K. M. Dyballa, R. Franke, S. R. Waldvogel, ChemistryOpen 2016, 5, 115-119. [14] T. Quell, N. Beiser, K. M. Dyballa, R. Franke, S. R. Waldvogel, Eur. J. Org. Chem. 2016, 2016, 4307-4310.



